上海金畔生物科技有限公司代理日本同仁化学试剂盒全线产品,欢迎访问日本同仁化学dojindo官网了解更多信息。
 11-HUPA
11-HUPA
11-HUPA

- 機能性有機材料
Self Assembled Monolayer(SAM)研究用試薬
-
製品コードH399 11-HUPA
-
CAS番号83905-98-0
-
化学名11-Hydroxyundecylphosphonic acid
-
分子式・分子量C11H25O4P=252.29
| 容 量 | メーカー希望 小売価格 |
富士フイルム 和光純薬 |
|---|---|---|
| 10 mg | ¥13,900 | 342-91603 |
- ご購入方法
- お問い合わせ
マニュアル
-
プロトコル
 金属酸化物表面にホスホン酸SAMを形成したい
金属酸化物表面にホスホン酸SAMを形成したい 
技術情報
注意事項
・本製品を粉末の状態で取り出し使用する場合、性状の性質上、静電気等の要因で容器内に付着し、取り出しにくい場合があります。
・容器内に付着し、取り出せなかった粉末に関しては、使用する溶媒を容器に入れ、溶かし出して使用してください。
参考文献
1) T. Hauffman, O. Blajiev, J. Snauwaert, C. van Haesendonck, A. Hubin, H. Terryn, "Study of the self-assembling of n-octylphosphonic acid layers on aluminum oxide", Langmuir, 2008, 24 (23), 13450.
2) P. Thissen, M. Valtiner, G. Grundmeier, "Stability of Phosphonic Acid Self-Assembled Monolayers on Amorphous and Single-Crystalline Aluminum Oxide Surfaces in Aqueous Solution", Langmuir, 2010, 26 (1), 156.
3) W. Gao, L. Reven, "Solid-state NMR-studies of self-assembled monolayers", Langmuir, 1995, 11 (6), 1860.
4) S. Marcinko, A. Y. Fadeev, "Hydrolytic Stability of Organic Monolayers Supported on TiO2 and ZrO2", Langmuir, 2004, 20 (6), 2270.
5) J. Schwartz, M. J. Avaltroni, M. P. Danahy, B. M. Silverman, E. L. Hanson, J. E. Schwarzbauer, K. S. Midwood, E. S. Gawalt, " Cell attachment and spreading on metal implant materials", J. Mat. Sci. Eng. C, 2003, 23, 395.
6) B. M. Silverman, K. A. Wieghaus, J. Schwartz, "Comparative properties of siloxane vs phosphonate monolayers on a key titanium alloy", Langmuir, 2005, 21 (1), 225.
7) N. Adden, L. J. Gamble, D. G. Castner, A. Hoffmann, G. Gross, H. Menzel, "Phosphonic Acid Monolayers for Binding of Bioactive Molecules to Titanium Surfaces", Langmuir, 2006, 22, 8197.
8) E. L. Hanson, J. Schwartz, B. Nickel, N. Koch, M. F. Danisman, "Bonding self-assembled, compact organophosphonate monolayers to the native oxide surface of silicon", J. Am. Chem. Soc. 2003, 125 (51), 16074.
9) M. Dubey, T. Weidner, L. J. Gamble, D. G. Castner, "Structure and Order of Phosphonic Acid-Based Self-Assembled Monolayers on Si(100)", Langmuir, 2010, 26 (18), 14747.
10) A. Vega, P. Thissen, Y. J. Chabal, "Environment-Controlled Tethering by Aggregation and Growth of Phosphonic Acid Monolayers on Silicon Oxide", Langmuir, 2012, 28, 8046.
11) P. Thissen, A. Vega, T. Peixoto, Y. J. Chabal, "Controlled, Low-Coverage Metal Oxide Activation of Silicon for Organic Functionalization: Unraveling the Phosphonate Bond", Langmuir, 2012, 28 (50), 17494.
12) J. T. Woodward, A. Ulman, D. K. Schwartz, "Self-assembled monolayer growth of octadecylphosphonic acid on mica", Langmuir, 1996, 12 (15), 3626.
13) A. Raman, M. Dubey, I. Gouzman and E. S. Gawalt, "Formation of self-assembled monolayers of alkylphosphonic acid on the netive oxide surface of SS316L", Langmuir, 2006, 22, 6469.
14) G. Zorn, R. Adadi, R. Brener, V. A. Yakovlev,| I. Gotman, E. Y. Gutmanas, C. N. Sukenik, "Tailoring the Surface of NiTi Alloy Using PIRAC Nitriding Followed by Anodization and Phosphonate Monolayer Deposition", Chem. Mater. 2008, 20, 5368.
15) R. Quinones and E. S. Gawalt, "Polystyrene formation on monolayer-modified nitinol effectively controls corrosion", Langmuir, 2008, 24, 10858.
16) S. C. D’Andrea and Al. Y. Fadeev, "Covalent surface modification of calcium hydroxyapatite using n-alkyl- and n-fluoroalkylphosphonic acids", Langmuir, 2003, 19, 7904.
17) B. Zhang, T. Kong, W. Xu, R. Su, Y. Gao and G. Cheng, "Surface functionalization of zinc oxide by carboxyalkylphosphonic acid self-assembled monolayers", Langmuir, 2010, 26(6), 4514.
18) A. Sharma, B. Kippelen, P. J. Hotchkiss and S. R. Marder, "Stabilization of the work function of indium tin oxide using organic surface modifiers in organic light-emitting diodes", Appl. Phys. Lett., 2008, 93, 163308.
19) Jing Shang, Fang Cheng, Manish Dubey, Justin M. Kaplan, Meghana Rawal, Xi Jiang, David S. Newburg, Philip A. Sullivan, Rodrigo B. Andrade, and Daniel M. Ratner, "An Organophosphonate Strategy for Functionalizing Silicon Photonic Biosensors", Langmuir, 2012, 28(6), 3338.
よくある質問
-
Q
ホスホン酸誘導体の溶解例を教えてください。
-
A
ホスホン酸誘導体の各溶媒への溶解性は以下の通りです。
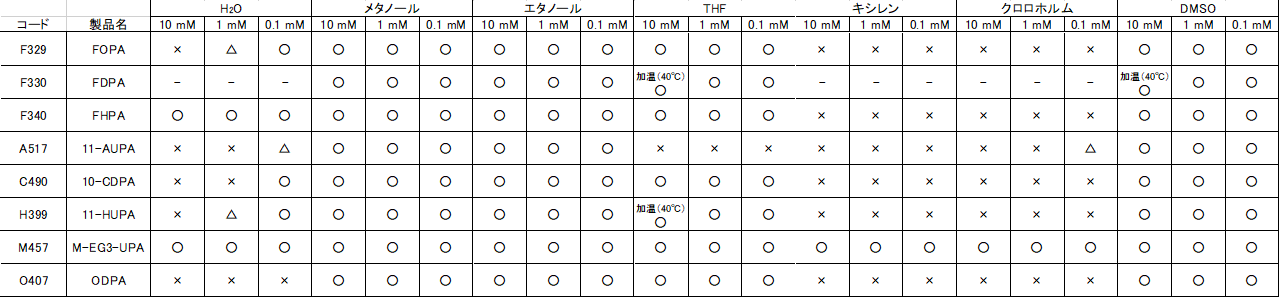


取扱条件
| 性状: | 本品は、白色~微黄色粉末である。 |
|---|---|
| ジメチルスルホキシド溶状: | 試験適合 |
| NMRスペクトル: | 試験適合 |


