上海金畔生物科技有限公司代理日本同仁化学 DOJINDO代理商全线产品,欢迎访问官网了解更多信息
上海金畔生物代理Hampton research品牌蛋白结晶试剂耗材工具等,我们将竭诚为您服务,欢迎访问Hampton research官网或者咨询我们获取更多相关Hampton research品牌产品信息。
Products > Optimize Reagents > Optimize – Salts > Trimethylamine N-oxide dihydrate
Trimethylamine N-oxide dihydrate
CAT NO
HR2-777
NAME
DESCRIPTION
100 mL
PRICE
$130.00
支持材料
 HR2-777 Trimethylamine N-oxide dihydrate SDS
HR2-777 Trimethylamine N-oxide dihydrate SDS应用
- Crystallization grade Trimethylamine N-oxide dihydrate for formulating screens or for optimization
- 结晶级三甲胺 N-氧化物二水合物,用于配制筛选或优化
特征
- Sterile filtered solution
- Formulated in Type 1+ ultrapure water: 18.2 megaohm-cm resistivity at 25°C, < 5 ppb Total Organic Carbon, bacteria free (<1 Bacteria (CFU/ml)), pyrogen free (<0.03 Endotoxin (EU/ml)), RNase-free (< 0.01 ng/mL) and DNase-free (< 4 pg/µL)
- 无菌过滤溶液
在 1+ 型超纯水中配制:25°C 时电阻率为 18.2 兆欧厘米,总有机碳 < 5 ppb,无细菌(<1 细菌 (CFU/ml)),无热原(<0.03 内毒素 (EU/ml)) , 无 RNase (< 0.01 ng/mL) 和无 DNase (< 4 pg/µL)
描述Trimethylamine N-oxide dihydrate
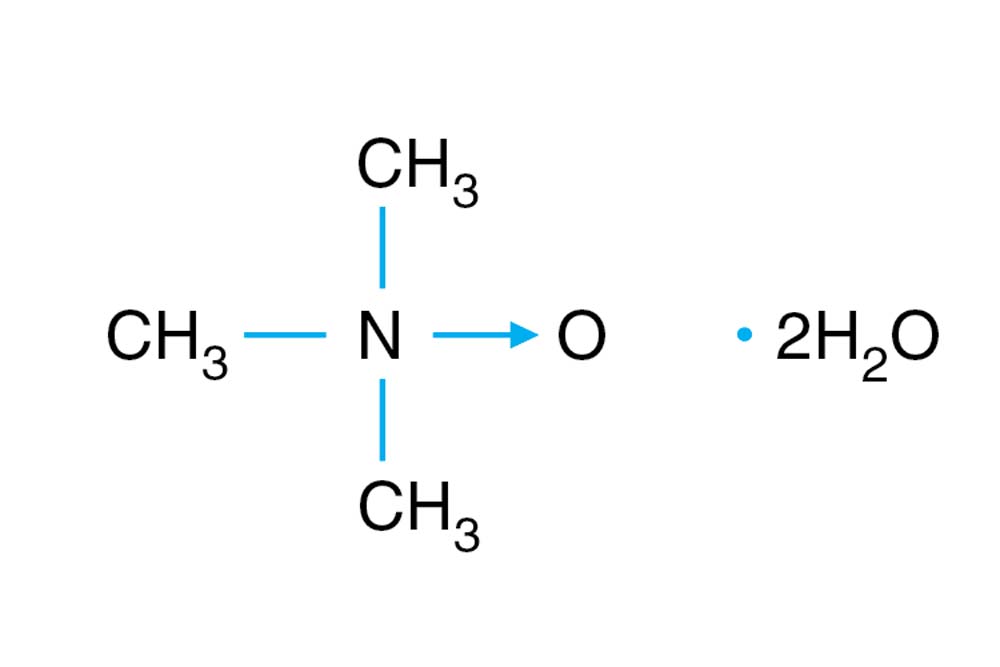
Synonym: TMANO
(CH3)3NO • 2H2O
C3H9NO • 2H2O
Mr 111.14
CAS Number [62637-93-8]
EC Number 214-675-6
Beilstein Registry Number 4(4)144
Merck 14,9711
RTECS YH2850000
MDL Number MFCD00002048
Purity > 98.0%
Maximum solubility is 6.0 M at 25°C (tested by Hampton Research)
Measured pH Range: 8.0 – 9.3 at 25°C
Measured Conductivity Range: 10.9 – 67.1 µS/cm at 25°C
Measured Refractive Index Range: 1.34320 – 1.34348 at 20°C
同义词: TMANO
(CH3)3NO • 2H2O
C3H9NO • 2H2O
111.14 先生
CAS 编号 [62637-93-8]
欧盟编号 214-675-6
贝尔斯坦注册号 4(4)144
默克 14,9711
RTECS YH2850000
MDL 编号 MFCD00002048
纯度 > 98.0%
25°C 时最大溶解度为 6.0 M(由 Hampton Research 测试)
测得的 pH 范围:25°C 时 8.0 – 9.3
测得的电导率范围:25°C 时为 10.9 – 67.1 µS/cm
测得的折射率范围:20°C 时为 1.34320 – 1.34348
Trimethylamine N-oxide dihydrate
参考
1. Use of organic cosmotropic solutes to crystallize flexible proteins: Application to T7 RNA polymerase and its complex with the inhibitor T7 lysozyme. Jeruzalmi and Steitz, J. Mol. Bio. (1997) 274, 748-756.
2. Trimethylamine N-oxide as a versatile cryoprotective agent in macromolecular crystallography. Mueller-Dieckmann, C., Kauffmann, B., and Weiss, M. J. Appl. Cryst. (2011). 44, 433–436.
3. Crystallization of a functionally intact Hsc70 chaperone. J. Jiang, E. M. Lafer and R. Sousa. Acta Cryst. (2006). F62, 39-43.
4. Doolittle, R. F. (2003). Biophys. Chem. 100, 307–313.
5. Hill, M. C., Bates, I. R., White, G. F., Hallett, F. R. & Harauz, G. (2002). J.
6. Auton, M. & Bolen, D. W. (2005). Proc. Natl Acad. Sci. USA, 102, 15065–15068.
7. Bolen, D. W. (2004). Methods, 34, 312–322.
